Transketolases
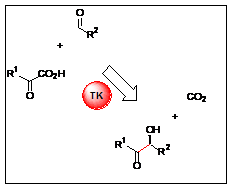
Chiral polyols synthesis: TK allow stereoselective preparation of (3S,4R)-ketoses catalysing an irreversible reaction using hydroxypyruvate (R1 = CH2OH) which is decarboxylated. This enzyme leads to different chiral derivatives, including non- commercially available phosphorylated sugars (D-sedoheptulose-7-P, D-xylulose-5-P…), halogenated sugars or antithrombotic thio-sugars.
Search for new catalytic activities: Our aims focus on substrate specificity modification of TK to enlarge its synthetic potential. New TK accepting different donor and acceptor substrates (R1 and R2 variables) and/or showing different stereoselectivity are screened in genomic banks (ANR genozyme). We also screen mutant enzyme banks (ANR deoTK) using screening or selection tests developed in our team. The screening tests are based on the decarboxylation of the donor substrate or on specific probe design leading to in vitro (fluorescence or amperometry) or in vivo detectable molecules under the catalytic action of TK. In the latter, the probes are L-Leu or L-Met precursors and auxotrophic cell growth (E.coli) is then directly correlated to TK catalytic activity.
Enzyme immobilisation (partnership with team MI): Immobilisation of enzymes are studied in materials like layered double hydroxides (LDH). These resulting biohybride materials allow syntheses optimisation (pH, T° and non conventionnal media resistance) in microfluidic reactors (ANR Nanacausys). The development of biocaptors based on the the co-immobilisation of TK with galactose oxydase or polyphenol oxydase is an other application for the detection of sugars or catalytic activities.

Search for new catalytic activities: Our aims focus on substrate specificity modification of TK to enlarge its synthetic potential. New TK accepting different donor and acceptor substrates (R1 and R2 variables) and/or showing different stereoselectivity are screened in genomic banks (ANR genozyme). We also screen mutant enzyme banks (ANR deoTK) using screening or selection tests developed in our team. The screening tests are based on the decarboxylation of the donor substrate or on specific probe design leading to in vitro (fluorescence or amperometry) or in vivo detectable molecules under the catalytic action of TK. In the latter, the probes are L-Leu or L-Met precursors and auxotrophic cell growth (E.coli) is then directly correlated to TK catalytic activity.

Enzyme immobilisation (partnership with team MI): Immobilisation of enzymes are studied in materials like layered double hydroxides (LDH). These resulting biohybride materials allow syntheses optimisation (pH, T° and non conventionnal media resistance) in microfluidic reactors (ANR Nanacausys). The development of biocaptors based on the the co-immobilisation of TK with galactose oxydase or polyphenol oxydase is an other application for the detection of sugars or catalytic activities.
