Aldolases
Aldolases: Chiral polyols synthesis: Fructose 6-phosphate aldolase (FSA) catalyses addition of dihydroxyacetone (R1 = CH2OH) donor on different aldehyde acceptors to give (3S,4R)-polyols. We have developed a highly stereoselective method for the preparation of aminocyclitols, glycosidase inhibitors potentially active on rare genetic lysosomal disorders. Unlike other aldolases, FSA is less specific towards its donor substrate allowing preparation of structurally diversified polyols (R1 = CH3, CH2CH3, H, CH2OH). This property has been exploited for the synthesis of rare and highly valuable monophosphorylated sugars. We also work on other aldolases presenting different stereoselectivities such as fuculose aldolase (3R,4R) or rhamnulose aldolase (3R,4S).
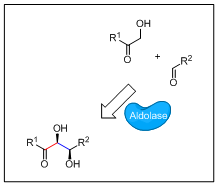
Search for new catalytic activities: New aldolases accepting different donor and acceptor substrates and/or showing different stereoselectivities are screened in genomic banks (ANR genozyme). We are also working on modifying catalytic FSA activity, FSA presents a robust and perfomant scaffold, using rational design mutagenesis methodologies.
Enzyme immobilisation (partnership with team MI): Immobilisation of enzymes are studied in materials like layered double hydroxides (LDH). These resulting biohybride materials allow syntheses optimisation (pH and non conventionnal media resistance) and development of bionanoreactors metabolic pathways inspired.

Search for new catalytic activities: New aldolases accepting different donor and acceptor substrates and/or showing different stereoselectivities are screened in genomic banks (ANR genozyme). We are also working on modifying catalytic FSA activity, FSA presents a robust and perfomant scaffold, using rational design mutagenesis methodologies.

Enzyme immobilisation (partnership with team MI): Immobilisation of enzymes are studied in materials like layered double hydroxides (LDH). These resulting biohybride materials allow syntheses optimisation (pH and non conventionnal media resistance) and development of bionanoreactors metabolic pathways inspired.
