Vous êtes ici : AccueilRechercheChimie Organique et MédicinalePeptoid
Thèmes de recherche
α,β-Peptoids

The synthesis of a novel family of peptidomimetics composed of linear and cyclic α,β-alternating peptoids was developed. Oligomers consisting of up to six peptoid residues (n = 1-3) were synthesized on large scale with use of an efficient iterative solution-phase method and longer oligomers (n = 4, 5) were obtained by the coupling of appropriately protected shorter oligomers. Preliminary conformational studies of these hybrid peptoids have been performed.
Convenient Solution-Phase Synthesis and Conformational Studies of Novel Linear and Cyclic α,β-Alternating Peptoids.
Hjelmgaard T., Faure S., Caumes C., De Santis E., Edwards A.A., Taillefumier C. Org. Lett. 2009, 11, 4100-4103.
Arylopeptoids
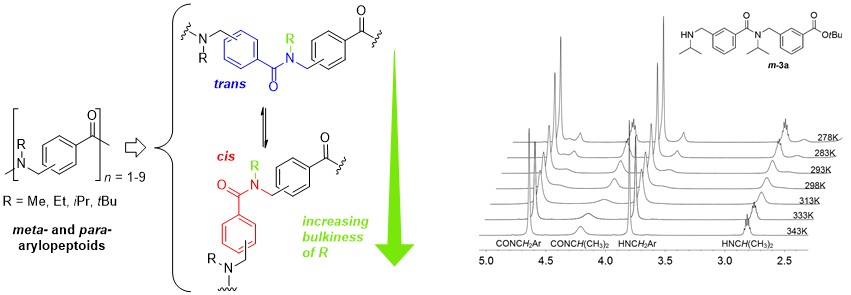
A highly convenient and efficient protocol for iterative solution phase synthesis of shorter oligomers of para- and meta-arylopeptoids (i.e. oligomeric N-substituted aminomethyl benzamides) was developed. Peptide coupling methods to access longer oligomers were studied and use of the new coupling reagent COMU was found to be the most efficient for creation of the tertiary benzamide bonds. The cis/trans isomerism of arylopeptoid backbones were studied by means of NMR and was found to be highly dependent on the nature of the side chains. Thus, increasing the bulkiness of the side chains would favor the cis amide bond conformation and arylopeptoids that carry tert-butyl side chains contain exclusively cis amide bonds.
Expedient Solution-Phase Synthesis and NMR Studies of Arylopeptoids.
Hjelmgaard T., Faure S., Staerk D., Taillefumier C., Nielsen J. Eur. J. Org. Chem. 2011, 4121-4132.
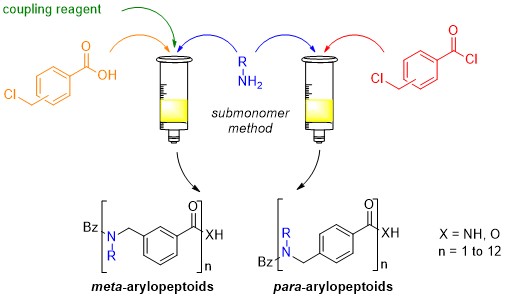
The solid-phase synthesis of para- and meta-arylopeptoids (oligomeric N-substituted aminomethyl benzamides) can be performed according two strategies involving a submonomer approach in which the arylopeptoid residues were created in an iterative manner on the growing chain using an acylation–substitution cycle. The first strategy involves in the acylation step, benzoic acid building blocks and the uronium salt COMU as efficient reagent for ensuring fast and clean coupling. An improved methodology was further developed using benzoyl chloride building blocks. This methodology has enabled the synthesis of arylopeptoids with tert-butyl and phenyl side chains, which allows for complete control over the amide conformation. The method has furthermore enabled the first synthesis and preliminary conformational studies of arylopeptoids bearing (S)-N-(1-phenylethyl) side chains.
Effect of capping groups at the N- and C-termini on the conformational preference of α,β-peptoids.
De Santis E., Hjelmgaard T., Caumes C., Faure S., Alexander B.D., Holder S.J., Siligardi G., Taillefumier C., Edwards A.A. Org. Biomol. Chem. 2012, 10, 1108-1122.
Improved solid-phase synthesis and study of arylopeptoids with conformation-directing side chains.
Hjelmgaard T., Faure S., De Santis E., Staerk D., Alexander B.D., Edwards A. A., Taillefumier C., Nielsen J. Tetrahedron 2012, 68, 4444-4454. (invitation, special issue of Tetrahedron (Symposium in print) on Chemistry of Foldamers).
Rapid and convenient semi-automated microwave assisted solid-phase synthesis of arylopeptoids.
Rasmussen J. E., Boccia M. M., Nielsen J., Taillefumier C., Faure S. Hjelmgaard T. Tetrahedron Lett. 2014, 55, 5940–5943.
Foldamers
Strategies are developed for the design of folded peptoids.
s1tbe : a chiral aliphatic side-chain
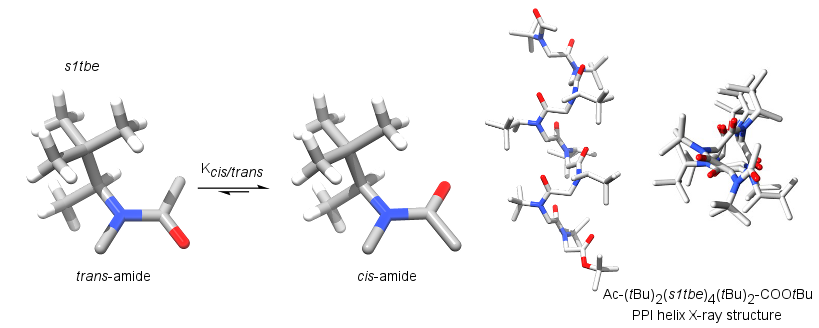
Homogeneous and robust Polyproline type I helices from peptoids with nonaromatic a-chiral side chains.
Roy O., Dumonteil G., Faure S., Jouffret L., Kriznik A., Taillefumier C. J. Am. Chem. Soc., 2017, 139, 13533–13540.
Tert-butyl : an achiral aliphatic side-chain
Dumonteil G., Bhattacharjee N., Gaetano A., Roy O., Faure S., Jouffret L., Jolibois F., Perrin L., Taillefumier C. J. Org. Chem. 2018, 83, 6382-6396.
Weak backbone CH...O=C and side chain tBu...tBu London interactions help promote helix folding of achiral NtBu peptoids.
Angelici G., Bhattacharjee N., Roy O., Faure S., Didierjean C., Jouffret L., Jolibois F., Perrin L., Taillefumier C. Chem. Commun., 2016, 52, 4573-4576.
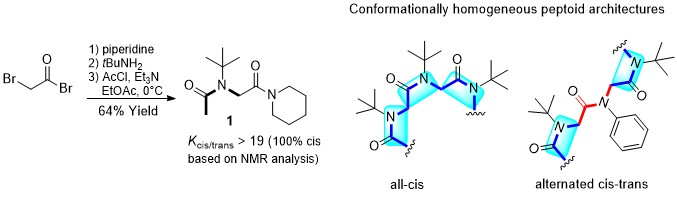
The very simple sterically hindered tert-butyl side chain exerts complete control over the peptoid amide geometry which only exists in the cis conformation. “All-cis” peptoids and alternating cis-trans peptoids have been constructed. The all-cis NtBu peptoid oligomers adopt a PolyProline-type I (PPI) helical conformation.
The Tert-Butyl Side Chain : A Powerful Means To Lock Peptoid Amide Bonds In The Cis Conformation.
Roy O., Caumes C., Esvan y., Didierjean C., Faure S., Taillefumier C. Org. Lett. 2013, 15, 2246-2249.
The triazolium-type side chains.
1,2,3-Triazolium-based peptoid oligomers.
Aliouat H., Caumes C., Roy O., Zouikri M., Taillefumier C., Faure S. J.Org. Chem., 2017, 82, 2386-2398.
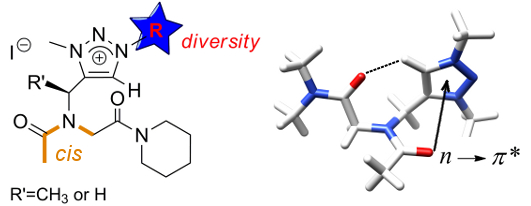
The positively charged triazolium-type side chain enables chemical diversity while enforcing the peptoid amide main chain to adopt the cis conformation. The cis conformation is primarily due to an attractive interaction arising from a backbone to side-chain n → π*Ar electronic delocalization.
The Click Triazolium Peptoid Side Chain : A Strong cis-Amide Inducer Enabling Chemical Diversity.
Caumes C., Roy O., Faure S., Taillefumier C. J. Am. Chem. Soc. 2012, 134, 9553-9556.
Biomimetic architectures
Cationic amphipathic peptide mimetics with antimicrobial activities.
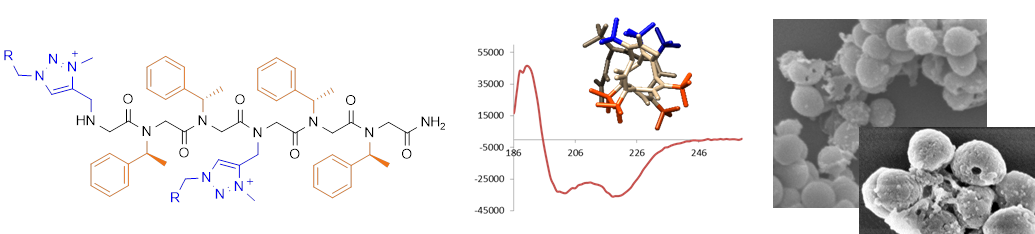
1,2,3-Triazolium-based cationic amphipathic peptoid oligomers mimicking antimicrobial helical peptides.
Shyam R., Charbonnel N., Job A., Blavignac C., Forestier C., Taillefumier C., Faure S. ChemMedChem, 2018, 13, 1513-1516.
Peptide hormone mimetics.
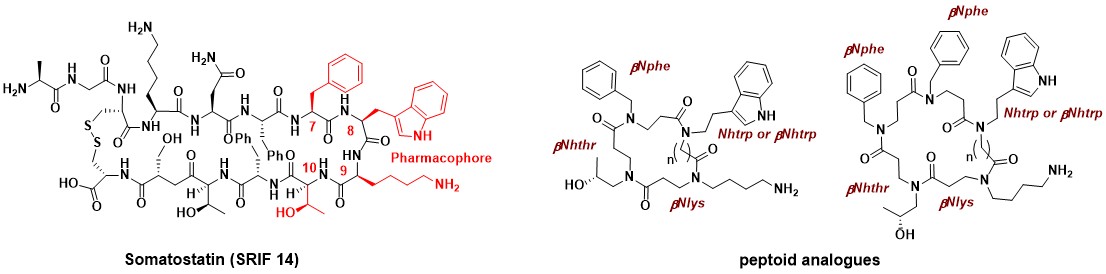
The synthesis and evaluation of the first “all-peptoid” SRIF (somatotropin release-inhibiting factor) analogues has been developed. This first generation of peptoids was designed to mimic the SRIF pharmacophore (Phe7-(D)Trp8- Lys9-Thr10). Cyclopeptoids have shown good affinity for the somatostatin subtype (sst) receptors sst3, sst4 and sst5 and lower potency for the sst1 and sst2 subtypes.
Synthesis And Binding Affinities For Sst Receptors Of Cyclic Peptoid SRIF-Mimetics
Caumes C., Hjelmgaard T., Roy O., Reynaud M., Servent D., Taillefumier C., Faure S. Med. Chem. Commun. 2012, 3, 1531-1535.
Macrocyclic peptoids
Cyclic β-peptoids
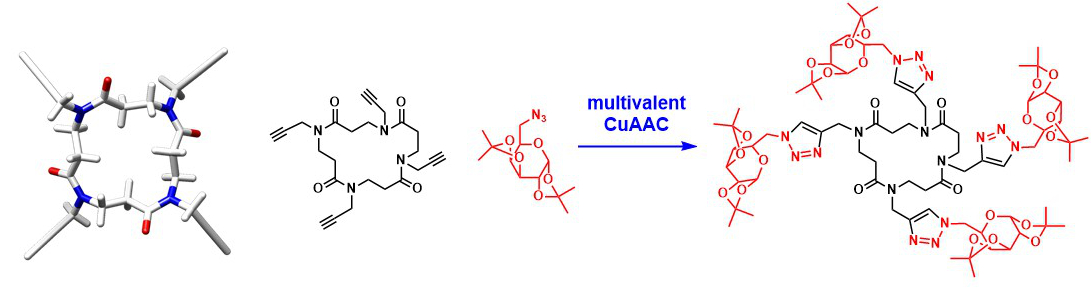
Cyclic β-peptoids.
Roy O., Faure S., Thery V., Didierjean C., Taillefumier C. Org. Lett. 2008, 10, 921-924.
Cyclic α,β-peptoids
Selective complexation of divalent cations by a cyclic α,β-peptoid hexamer: a spectroscopic and computational study.
De Santis E., Edwards A. A., Alexander B. D., Holder S. J., Biesse-Martin A.-S., Nielsen B. V., Mistry D., Waters L., Siligardi G., Hussain R., Faure S., Taillefumier C. Org. Biomol. Chem., 2016, 14, 11371-11380.
Cyclic α,β-Tetrapeptoids : Sequence-Dependent Cyclization And Conformational Preference
Caumes C., Fernandes C., Roy O., Hjelmgaard T., Wenger E., Didierjean C., Taillefumier C., Faure S. Org. Lett. 2013, 15, 3626-3629.
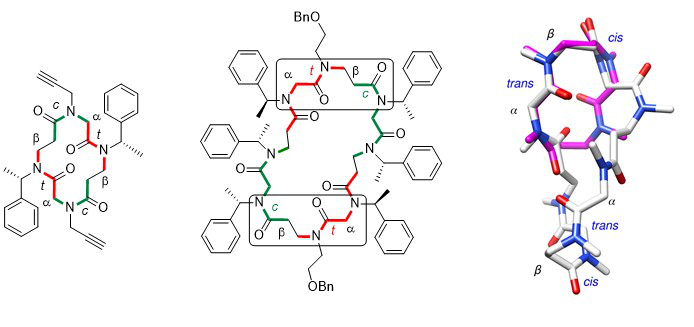
The comparison of the crystal structures of a cyclic α,β-tetramer and a cyclic α,β-octamer reveals striking similarities. Indeed, both structures have the presence of two αtrans-βcis segments in a turn-like conformation in common. The fact that this specific conformation is also present in an unconstrained cyclic octamer, having a different side chain sequence, suggests that the observed turn may represent a privileged conformation of the α,β-peptoid family.
Cyclic α,β-peptoid octamers with differing side chain patterns : synthesis and conformational investigation.
De Santis E., Hjelmgaard T., Faure S., Roy O., Didierjean C,. Alexander B.D., Siligardi G., Hussain R., Javorfi T., Edwards A.A., Taillefumier C. Amino Acids 2011, 41, 663-672.
Cyclic arylopeptoids
Topologically Diverse Shapes Accessible by Modular Design of Arylopeptoid Macrocycles.
Hjelmgaard T., Nauton L., De Riccardis F., Jouffret L., Faure S. Org. Lett., 2018, 20, 268-271.
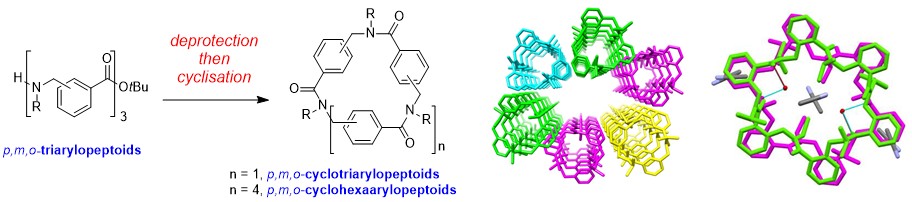
The head-to-tail conversion of linear arylopeptoids (oligomeric N-substituted aminomethyl benzamides) into the derived novel macrocycles has enabled the first X-ray structures of arylopeptoid constructs and the identification of well-defined architectures in solution.
Macrocyclic Arylopeptoids – A Novel Type Of Cyclic N-Alkylated Aromatic Oligoamides Forming Nanotubular Assemblies
Hjelmgaard T., Roy O., Nauton L., El-Ghozzi M., Avignant D., Didierjean C., Taillefumier C., Faure S. Chem. Commun. 2014, 50, 3564-3567.
Multivalent peptoid architectures
Multivalent glycopeptoids (linear and cyclic) are being currently developed to obtain high-affinity ligands for lectin receptors.
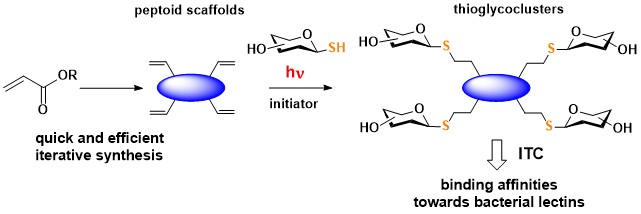
Solution-phase synthesis of linear and cyclic β- and α,β-peptoids was coupled to photo-induced thiol–ene coupling (TEC) reactions to readily access multivalent thioglycoclusters. A tetrameric cyclic β-peptoid scaffold displaying 1-thio-β-D-galactose or 1-thio-α-D-mannose has revealed efficient binding potency for bacterial lectins LecA and BC2L-A.
Multivalent thioglycopeptoids via photoclick chemistry : potent affinities towards LecA and BC2L-A lectins
Caumes C., Gillon E., Legeret B., Taillefumier C., Imberty A., Faure S. Chem. Commun. 2015, 51, 12301-12304.
From Glycopeptides to Glycopeptoids.
Szekely T., Roy O., Faure S., Taillefumier C. Eur. J. Org. Chem. 2014, 5641–5657.
Selectivity among Two Lectins : Probing the Effect of Topology, Multivalency and Flexibility of "Clicked" Multivalent Glycoclusters.
Cecioni S., Faure S., Darbost U., Bonnamour I., Parrot-Lopez H., Roy O., Taillefumier C., Wimmerova M., Praly J.P., Imberty A., Vidal S. Chem. Eur. J. 2011, 17, 2146-2159.
Click glycoconjugation of per-azido- and alkynyl-functionalized β-peptides built from aspartic acid.
Barra M., Roy O., Traikia M., Taillefumier C. Org. Biomol. Chem. 2010, 8, 2941-2955.
X-ray structures
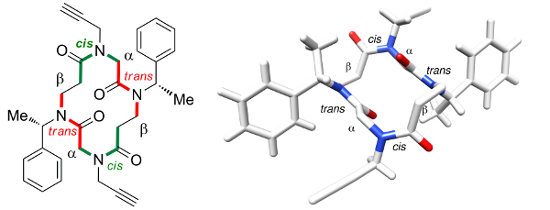
Cyclic α,β-Tetrapeptoids : Sequence-Dependent Cyclization And Conformational Preference
Caumes C., Fernandes C., Roy O., Hjelmgaard T., Wenger E., Didierjean C., Taillefumier C., Faure S. Org. Lett. 2013, 15, 3626-3629.
Fichier CIF
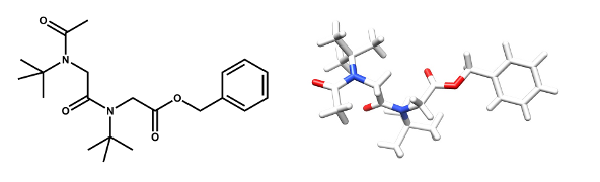
The Tert-Butyl Side Chain : A Powerful Means To Lock Peptoid Amide Bonds In The Cis Conformation.
Roy O., Caumes C., Esvan y., Didierjean C., Faure S., Taillefumier C. Org. Lett. 2013, 15, 2246-2249.
Fichier CIF





 ENT
ENT Annuaire
Annuaire Se connecter
Se connecter